Trodelvy的3期临床达到主要终点,对短期股价的影响却是负面的,下面看下吉利德的投资者问答了解下这次事件。
1. 新闻稿
2. 投资者问答
3. 结语
1
新闻稿
3月7日,吉利德公司公布了3期临床TROPiCS-02的研究结果,该研究评估Trodelvy对比医生选择的化疗,治疗HR+/HER2-、既往接受过内分泌疗法、CDK4/6抑制剂和两到四线化疗的转移性乳腺癌患者。
该研究达到了主要终点,与医生选择的化疗相比,PFS有统计学意义的改善,将疾病进展或死亡的风险降低30%。对关键次要终点OS的第一次中期分析表明,OS有改善的趋势,将继续跟踪患者的OS情况。Trodelvy的安全性与之前的研究一致。
2
投资者问答
Q1:Did PFS meet your bar for clinically meaningful?
回答:There is a broad range of views on what is “clinically meaningful” in this population. We are evaluating the data and will explore potential pathways with regulatory authorities to bring Trodelvy to this group of patients.
评论:以PFS作为单一指标去衡量临床意义是偏面的,在达到统计学上的差异后,此时还应该考虑生存情况以及生活质量。
Q2:You reference TROPiCS-02 clinical activity consistent with the Phase 1/2 IMMU-132-01 study. What were the results of that study?
回答:The Phase 1/2 IMMU-132-01 study evaluated Trodelvy in a subset of HR+/HER2- metastatic breast cancer patients. As published here [at 网页链接(20)42445-7/fulltext] (Kalinsky et al, 2020), a PFS of 5.5 months was reported. There was no comparator arm in this study.
评论:这时候参照过去的单臂试验数据意义不大,只要Trodelvy打败这次的化疗对照组,甭管这次PFS/OS是略微大于还是小于之前的单臂数据。
Q3:What does this mean for HR+/HER2- earlier line patients?
回答:We saw statistically significant clinical activity in TROPiCS-02, and there is no change to our plan to move forward with studies for earlier line patients.
评论:向前线推进计划不变,但潜在人群空间到底会有多大?目前HR+/HER2-乳腺癌人群的推荐疗法以内分泌疗法和靶向疗法为主,只有那些肿瘤负荷大、出现危及生命的内脏转移、需要快速控制病情的,或HR+内分泌治疗失败或耐药等,才需要用到化疗,这类群体到底占有多少?同时Trodelvy是不太可能与内分泌疗法和靶向疗法(CDK4/6等)竞争同样的人群,未来的对照组依旧是化疗药,带靶的化疗药会打不过普通的化疗药?
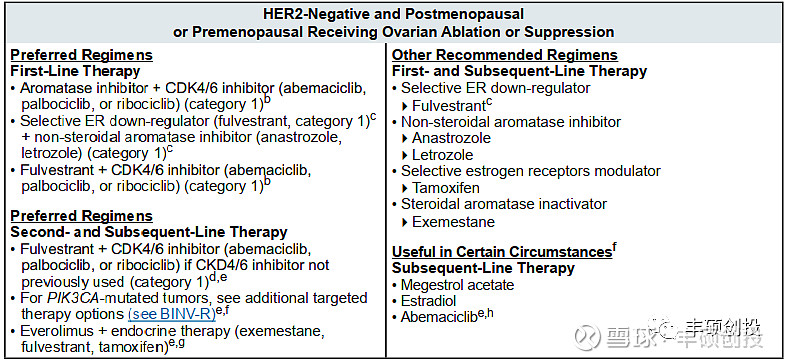
图1 NCCN 2022 V2指南
Q4:What are the next milestones for this trial?
回答:The final overall survival data readout is expected in 2024, subject to the timing of events.The study allows for a second, interim OS analysis and we will update you on our expectations following review with regulatory authorities.In the meantime, patients will continue to be followed and treated per protocol.
评论:OS数据方面最晚于2024年公布,这是该项试验的最后一次节点。之前可以有第二次的OS中期分析,到时候可以与FDA更新沟通,如果结果不错,估计离获批也不远了。
Q5:Is it reasonable to expect a statistically significant final overall survival readout given the data so far?
回答:In the first interim analysis, the study demonstrated a trend in improvement for overall survival which means Trodelvy is showing a numerical improvement that was not statistically significant at the first interim analysis. We will continue to follow patients while the data matures to understand the final overall survival result.
评论:这一次的中期分析OS并没有达到统计学意义上的差异,虽然具有改善的趋势。数据仍未成熟,后续OS结果如何仍需等待。显然由于这点,投资者未能提前摘取果实,对股价造成了负面波动。
Q6:How does this impact Gilead’s 2022 guidance?
回答:We are not changing our 2022 guidance at this time.In terms of the 2022 revenue guidance issued on February 1st, we did not assume any TROPiCS-02-related launches until late 2022 at the earliest.We expect an immaterial impact to Operating Expenses in 2022 with the trial still ongoing and our planned studies on-track.We are evaluating the impact to our in-process R&D on the balance sheet, and that could impact our GAAP EPS guidance. We expect to update you on our Q1 call.
评论:计划没变,但关于这项试验的研发费用情况对资产负债表的影响将在Q1季报介绍最新情况。计划没变却扯上费用的事,这一切缘由也许与2021年4月21日的一次变化有关。那天吉利德在clinicaltrials更新了TROPICS-02试验信息,其中招募人数从2019年登记的400人扩展到543人。招募人数多了,费用上来了,继而影响了EPS。其实这次招募人数的变化,也有点透露了吉利德对这项试验的信心变化,这是统计学上的理解,也幸亏这次招募人数的扩展,不然TROPICS-02失败的可能性会增加。
Q7:Is there any change to your goal that 1/3 of Gilead’s revenue will derive from oncology products in 2030?
回答:We remain confident in that goal.The 2030 goal was risk-adjusted and – given the nature of our business – allowed for a range of outcomes across our portfolio.
评论:无。
Q8:Are there any changes to the Trodelvy program?
回答:We are very confident in Trodelvy’s activity and pan-tumor potential given ASCENT trial data for mTNBC and TROPHY U-01 for mUC – and the approvals that followed – in addition to TROPiCS-02 which showed statistically significant PFS.TROPiCS-02 is ongoing, in addition to a range of other Trodelvy trials, including 1L TNBC and NSCLC in combination with Keytruda, or in our other ongoing later-line trials in bladder cancer and NSCLC. Most of the 20+ oncology trials we plan to initiate this year include Trodelvy.
评论:Q8和Q7的回答用词不一样,对Trodelvy这个项目是“very confident”,有冲刺扩张的意思。而对2030年的1/3收入来自肿瘤是“remain confident”,是稳步前进的意思。
结语
从1/2期IMMU-132-01研究结果中,看到Trodelvy对有无经过CDK4/6i疗法的患者群体的效果是差别较大的。未接受过CDK4/6i的组的mPFS和mOS分别为7.6个月和21.7个月,接受过CDK4/6i的组的mPFS和mOS分别为3.8个月和11.0个月。而TROPICS-02试验则全是接受过CDK4/6i的患者,虽说只要比化疗对照组优效即行,但在一个对化疗药物十分不友好的群体里,无疑会拉低化疗药的效果,也就是增大组间做出差异的难度。这次达到主要终点(PFS)是一个里程碑事件,即使在这么一个恶劣的环境下,带靶的化药依然是优于普通化药的,期待后续的更多数据。
声明:以上内容仅供参考,不构成投资建议。
丰硕创投
丰硕创投成立于2019年,专注于大健康领域的投资。丰硕创投是一家以基本面研究为基础的创投,注重公司长期发展价值的挖掘。通过多维度的认知和不断自我进化构建完整的投资体系。我们有受托人基因,对资本市场有敬畏之心,把风险控制放在首位,用狙击式打法和专业的视野持续为投资人创造价值。