

$复星医药(SH600196)$ $复星医药(02196)$ @曾经的sw @stocktone @Clover_ @淡定九点半
一、企业概况:

二、管理团队:(人员、数据截至到2014年3月)
Simon Allen:(CBO)
Education:University of Sydney
Last serving as the Chief Executive Officer of Kalypsys, Mr. Allen has held increasing levels of responsibility at companies such as CovX, Nuvelo, SkyePharma and Gilead.
He graduated from the University of Sydney with a B.S. and earned his M.B.A. from the Australian Graduate School of Management.
Served:
1、Gilead Sciences(team Member);
2、SkyePharma(team Member);
3、Nuvelo(team Member);
4、Covx(team Member);
5、Kalypsys(CEO)
Lawson Macartney:(CEO)
Education:University of Glasgow
Mr.Lawson Macartney spent nearly 20 years working with GlaxoSmithKline (GSK) where he most recently served as Senior Vice President of Global product Strategy and Project/Portfolio Management, responsible for global commercial functions.
Throughout his time at GSK, Dr Macartney held leadership roles in the Cardio-metabolic and Urology therapeutic area, from project leader to Global head for this extensive franchise.
Prior to his experience at GSK, he worked at AstraMerck and Astra Pharmaceuticals in leadership roles in operations, Marketing and Sales.
Served:
1、Astra Pharmaceuticals;
2、GlaxoSmithKline:
——Project Leader to Global Head for this Extensive Franchise
——Leadership Roles in the Cardio-Metabolic and Urology Therapeutic Area
——Senior Vice President of Global Product Strategy and Project/Portfolio Management
3、Shire
Ho Sung Cho:(CTO)
Dr. Cho has over 20 years of experience in recombinant protein expression, purification, modification, and analysis. He was a staff scientist at Lawrence Berkeley National Labs where he worked on the structural genomics project, applying NMR spectroscopy and X-ray crystallography to determine structures of proteins at atomic resolution
Served:
1、Burroughs Wellcome(Medicinal Chemist in the Oncology Department);
2、Lawrence Berkeley National Laboratory(Staff Scientist);
3、Exelixis(Team Leader);
4、Xencor(Associate Director of Protein Chemistry)
Peter Kiener:(CSO)
Education:Lancaster University
Dr. Kiener is currently the CEO of Zyngenia, Inc. and is the Chairman of the Board of Managers of Resolve Therapeutics, LLC. Dr. Kiener's most recent position was at AstraZeneca PLC/MedImmune, LLC as executive vice president and global head of biologics R&D.
Dr. Kiener received his B.A. in Chemistry from the University of Lancaster, UK, and his D. Phil. in Biochemistry from Oxford University, UK. Dr. Kiener has published more than 100 papers in peer-reviewed journals, and is an inventor on more than 20 patents and patent applications.
Served:
1、Bristol-Myers Squibb(Team Member);
2、MedImmune(Vice President and Head of Research and Development );
3、AstraZeneca( Vice President and Head of Research and Development );
4、Zyngenia(President & CEO and Co-Founder)
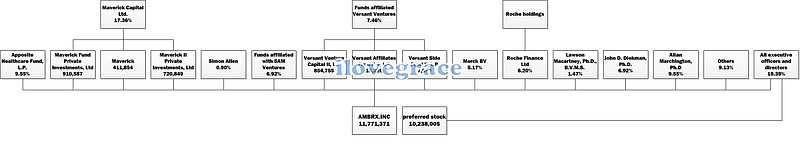
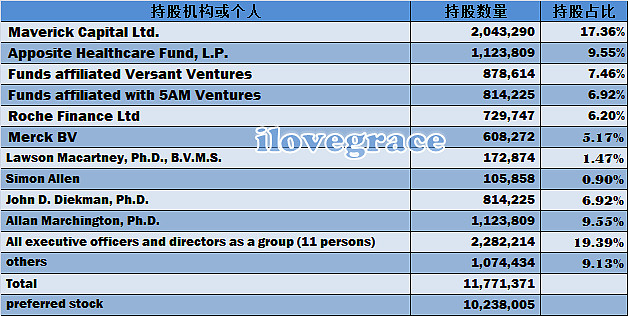
三、股权结构:
四、部分财务数据:
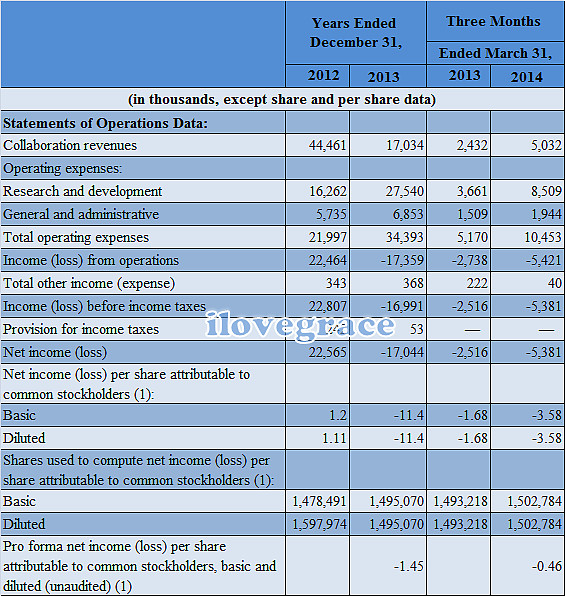
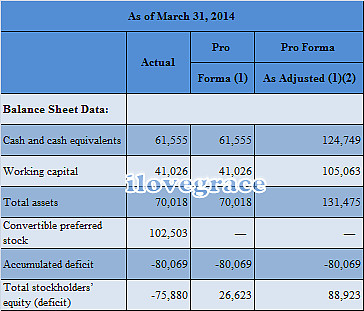
五、研发进展:
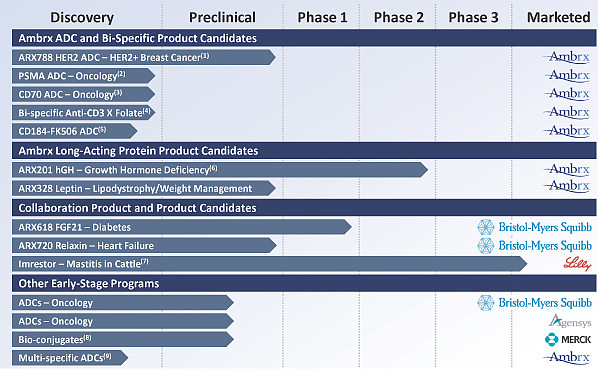
六、合作方:

In April of 2013, Astellas Pharma Inc. entered into a collaboration with Ambrx Inc. for the discovery and development of novel antibody drug conjugates (ADCs). ADCs allow for the targeted delivery of drugs to the target tissue. Ambrx creates optimized ADCs using its site-specific conjugation technology along with proprietary linkers and payloads. In the preclinical setting, Ambrx ADCs have demonstrated high potency and a wider therapeutic index than ADCs created using conventional non-specific conjugation.
In January 2007, we entered into a collaborative research, license and commercialization agreement with Eli Lilly, pursuant to which we work with Eli Lilly’s Elanco Animal Health division to discover, develop and commercialize a broad portfolio of Ambrx-enabled bio-conjugates for animal health. In February 2011 this agreement was amended. Under this agreement, as amended to date, we granted Eli Lilly an exclusive, worldwide license to utilize certain of our drug technology, trade secrets, know-how, patent rights and inventions to develop and commercialize certain product candidates in the field of animal health, as well as human and non- human food safety applications.
In September of 2011, Ambrx entered into a worldwide collaboration with Bristol-Myers Squibb to develop and commercialize product candidates surrounding the Fibroblast Growth Factor 21 (FGF-21) protein, for potential use in treating type 2 diabetes, and the Relaxin hormone, for potential use in treating heart failure. Derivatives of FGF-21 and Relaxin were developed using Ambrx’s unique ReCODE™ platform technology to modify the native proteins with amino acid building blocks beyond the 20 naturally occurring amino acids. Bristol-Myers Squibb and Ambrx also entered into research collaborations for both programs, which focus on engineering enhanced versions of the two target proteins for therapeutic use. In May of 2013, Ambrx entered into a collaboration agreement with Bristol-Myers Squibb for the discovery and development of novel antibody drug conjugates using Ambrx’s Protein Medicinal Chemistry™ technology. These drug candidates will be based on Ambrx’s EuCODE™ technology.

In April of 2014 Ambrx entered into a co-development and license agreement with Zhejiang Hisun Pharmaceutical Co., Ltd. or Hisun, for the research, development and commercialization of certain bi-specific product candidates and products for the treatment of cancer. Hisun will have commercial rights to the products in China, while Ambrx will retain commercial rights outside of China and will be entitled to receive royalties on sales of the products in China.

In June of 2012, Ambrx entered into a collaboration with Merck to design and develop rationally optimized biologic drug conjugates based on Ambrx’s site-specific protein medicinal chemistry technology. By combining the targeting properties of biologics with the potent therapeutic properties of small molecules, Merck and Ambrx plan to design and optimize new ways to specifically deliver pharmacologically active compounds to their site of action while minimizing the potential for systemic effects.

In June of 2013 Ambrx entered into a collaboration with Zhejiang Medicine Co., Ltd. to develop and commercialize ARX788, Ambrx’s most advanced internally developed site-specific ADC targeting HER2-positive breast cancer. Under the agreement, Ambrx and ZMC will continue the development of ARX788. ZMC received commercial rights in China while Ambrx retained commercial rights outside of China and receives royalties on sales of the product in China.