$阿斯利康(AZN)$ $吉利德科学(GILD)$ TROP2 ADC dato Dxd 2L+ non-sq NSCLC vs 多西他赛 ,OS 优势未达到统计学显著性,有临床意义。2L 多西确实不太容易打败。网页链接
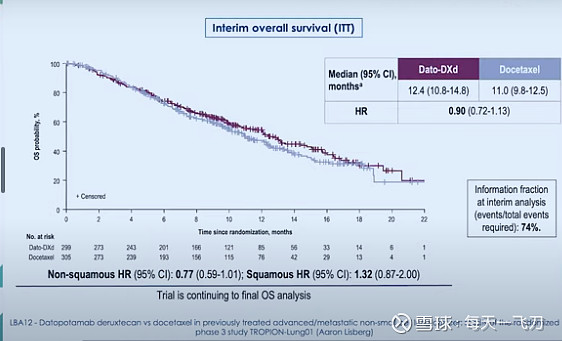
下图是去年ESMO中期分析结果
| 发布于: 修改于: | 雪球 | 转发:0 | 回复:24 | 喜欢:5 |
$阿斯利康(AZN)$ $吉利德科学(GILD)$ TROP2 ADC dato Dxd 2L+ non-sq NSCLC vs 多西他赛 ,OS 优势未达到统计学显著性,有临床意义。2L 多西确实不太容易打败。网页链接
下图是去年ESMO中期分析结果


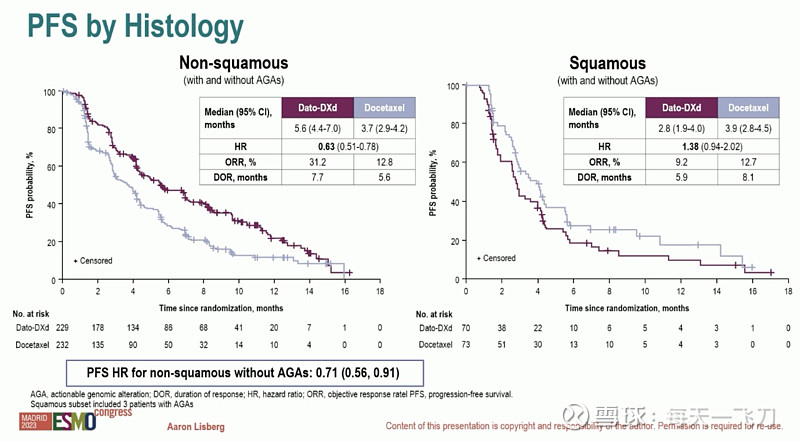
我重新读了一下,看了一下wording,很可能non sq人群 HR上限是<1的,达到数值上的统计显著性。但由于预设的是全人群的分析,所以PR在non sq 亚组中不能claim stat significant,可以看对于PFS的写法。
datopotamab deruxtecan demonstrated a statistically significant improvement in PFS in the overall trial population and a clinically meaningful PFS benefit in patients with nonsquamous NSCLC.
所以在non sq 人群疗效可能不错,等ASCO数据。
应该是ITT人群 OS HR 0.90,没有达到统计学意义;NSQ人群OS HR 0.77,有临床意义的改善;
申报上市针对的是【既往接受过全身治疗的局部晚期或转移性非鳞状NSCLC患者】
官宣了么
乳腺癌也是pfs获益,os估计也悬
In the overall trial population, survival results numerically favoured AstraZeneca and Daiichi Sankyo’s datopotamab deruxtecan but did not reach statistical significance
TROPION-Lung01 previously met the dual primary endpoint
of progression-free survival in the overall trial population
Results support applications currently under review by
regulatory authorities globally including in the US and EU