ASCO信息:
康宁杰瑞
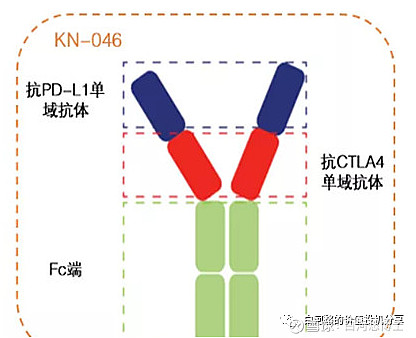
和2021年9月ESMO年会上披露的摘要相比,有以下信息值得注意:
入组患者从25人增加至55人,可评估患者从21人增加至52人,已完成所有计划招募患者入组,以后可以等待OS数据不断成熟;
2、ORR从57%变为51.9%,略微下降,但明显高于IMbrave150研究中阿替利珠+贝伐单抗的ORR(29.8%)、RESCUE研究中一线治疗组卡瑞利珠+阿帕替尼的ORR(34.3%);
3、披露生存期数据,mPFS为9.3个月,明显长于3期IMbrave150研究中阿替利珠单抗+贝伐单抗的mPFS(6.8个月)、2期RESCUE研究中一线治疗组卡瑞利珠单抗+阿帕替尼的mPFS(5.7个月)、3期ORIENT-32研究中信迪利单抗+贝伐单抗类似药的mPFS(4.6个月);
4、“3级及以上治疗相关不良反应发生率”从8%飙升至27.3%,“治疗相关死亡率”从0%飙升至7.2%。安全性从“可接受(accepted)”变为“可管理(manageable)”

点评:数据很好,安全性吓人!、3人因不良反应停药,4人死于不良反应。
如果死亡4人均由KN046所致,它的安全性还能称得上可管理(manageable)?
可以清晰的看到康宁周五一路下跌伴随着康方的一路上涨,这两可是主要竞争关系。
加科思:
As of January 28th, 2022, 53 patients with a median age of 62 years (39-79) were enrolled in 5 different dose levels: 200mg QD, 400mg QD, 800mg QD, 400mg BID and 400mg TID. Most patients (55%) had ≥ 2 prior lines of therapy. No dosing-limiting toxicities were observed. Two treatment-related adverse events (TRAEs) were G4 neutropenia (1 in 400mg BID and 1 in 400mg TID). The most common TRAEs (≥ 10%) included anemia (24.5%), total bilirubin increase (20.8%), direct bilirubin increase (15.1%), proteinuria (13.2%) and indirect bilirubin increase (11.3%). Only Grade 1 and 2 TRAEs were observed in the QD cohorts. A total of 33 patients (22 NSCLC, 9 CRC and 2 pancreatic cancer) had at least 1 post-baseline tumor assessment; in the 800mg QD cohort, overall response rate (ORR) and disease control rate (DCR) were 50% (5/10) and 100% (10/10), respectively, including 4 non-confirmed partial response (PR); in the 400mg QD cohort had an ORR and DCR of 80% (4/5) and 100% (5/5) respectively, including 2 non-confirmed PR. Patients with NSCLC (400mg QD and 800mg QD), the ORR and DCR were 70% (7/10) and 100% (10/10), respectively, including 5 non-confirmed PR. With respect to the pharmacokinetics analysis, JAB-21822 was rapidly absorbed with an average Tmax of 2 hr and reached higher plasma exposures (Cmax and AUC0-24h) after a single dose and multiple doses at C1D8.
看着还不错,有效性不太有可比性、但纸面上400mg QD和800mg QD组里NSCLC的ORR有7/10,QD组只有一二级TRAE、BID和TID组各有1例四级TRAE。
的KRAS G12C基本凉了,也会对加科思产生遐想。

君实生物:
2022年5月27日,ASCO官网公布了本届大会入选摘要的详细信息,先来一睹为快君实生物肿瘤领域产品注册研究摘要精彩详情:Icatolimab(TAB004/JS004)是君实生物自主研发一种特异性针对B-和T-淋巴细胞衰减因子(BTLA)的重组人源化IgG4单克隆抗体,其与特瑞普利单抗类似,在重链铰链区228号丝氨酸蛋白位点引入了脯氨酸(S228P)点突变,增加抗体的稳定性,减少IgG4可变区置换。
在前期的体外和体内研究表明,Icatolimab可结合 BTLA 并阻断其与其配体 HVEM 的相互作用,促进肿瘤特异性T淋巴细胞增殖和提高淋巴细胞功能,减轻BTLA人源化小鼠的肿瘤模型肿瘤负荷并提高存活率。同时,研究显示,BTLA 和 PD-1 通路的共同阻断能够进一步改善抗原特异性 T 细胞应答,为抗肿瘤免疫治疗联合方案提供了新的可能。
作为全球首个进入临床开发阶段的抗肿瘤抗BTLA单抗,在本届ASCO年会上,Icatolimab首次公布了I期临床试验结果。编号 2643
抗 BTLA 抗体 icatolimab 单药治疗晚期实体瘤患者的 Ia 期剂量递增研究
Phase Ia dose-escalation study of the anti-BTLA antibody icatolimab as a monotherapy in patients with advanced solid tumor.
主要研究者
Russell J. Schilder, MD,Sidney Kimmel Cancer Center, Thomas Jefferson University
该项剂量递增研究首次在人体中评估icatolimab单药治疗晚期实体瘤患者的初步安全性和有效性的(NCT04137900)。Icatolimab 以0.3 mg/kg、1 mg/kg、3 mg/kg和 10mg/kg (静脉滴注,Q3W)进行剂量递增,随后在 3 mg/kg和 10mg/kg 中剂量扩展,直至疾病进展或出现无法耐受的毒性。剂量限制毒性 (DLT) 由安全性监察委员会进行评估。研究终点包括安全性、药代动力学、药效学和抗肿瘤活性。
截至 2021 年 12 月 31 日,该项研究递增阶段共纳入25 例晚期实体瘤患者,中位年龄为 62(范围 32-85)岁, 16 例(64%)为男性患者,先前接受治疗线中位数为4, 15 例患者 (60%) 为PD-1/L1 治疗后进展。中位随访时间为 32 周。
抗肿瘤活性:
a)在19 例可评估患者中,研究者根据 RECIST v1.1标准评估,观察到 1 例(黑色素瘤)部分缓解(PR)和 6 例疾病稳定( SD);
b)该例PR的黑色素瘤患者,先前接受过纳武利尤单抗和 BRAF/MEK 抑制剂治疗,在接受icatolimab后持续缓解已超过 12 个月。
在 3 mg/kg和 10 mg/kg 队列中均观察到BTLA 受体被充分占位。
在递增的4个剂量组中,icatolimab 的平均半衰期为 7.5 ~ 19.2 天。
生物标志物初步分析表明, HVEM 和 CD8 的共表达与良好的缓解可能相关。
安全性方面,未观察到 DLT。24 (96%) 例患者发生治疗期间不良事件 (TEAE),其中 7例 (28%) 为3 级 TEAE,未发生≥ 4 级TEAE。AE 发生率或严重程度与剂量无关。最常见的 TEAE 包括疲劳 (32%)、腹痛 (20%)、腹泻 (16%)、关节痛 (16%)、天冬氨酸氨基转移酶升高 (16%)、便秘 (16%) 和挫伤 (16%)。1例(4%)患者因 TEAE停药。4例 (16%) 患者出现免疫相关 AE。
亚盛医学:
HQP1351
On the cutoff date of January 30, 2022, 39 pts (median age 52 [range 19-72] years) had received at least 1 dose of olverembatinib. The average (range) treatment period was 5.0 (0.2-35) months. PK analyses indicated an approximately dose-proportional increase in systemic exposure over the dose range of 20 to 50 mg. Thirty-one pts had KIT or PDGFRA mutations, 13 had stable disease for at least 2 cycles as the best response, 8 withdrew early, and 10 had progressive disease before Cycle 3. Very interestingly, 6 of 8 pts with KIT wild-type GIST were confirmed as SDH deficient: 2 pts had partial responses (PRs), 1 patient’s tumor had shrunk by 35.9% and lasted for 16 cycles, and another patient’s tumor had shrunk by 54.2% in the first evaluation. Four pts had stable disease as best response for 2, 6, 14, and 36 cycles. A total of 36 (92.3%) pts experienced treatment-emergent adverse events, most of which were mild or moderate. Ten (25.6%) pts experienced serious adverse events, of which intestinal obstruction attributed to GIST was the most reported. Common treatment-related adverse events (≥ 20%) included increased leukocyte (59.0%) and neutrophil (46.2%) counts, anemia (20.5%), constipation or asthenia (35.9% each), hyperuricemia (25.6%), hypoalbuminemia (23.1%), and elevated AST or ALT (20.5% each).
AK112
133 pts were enrolled from Feb 03, 2021 to Dec 31, 2021 and received AK112 plus chemotherapy (44 received AK112 10 mg/kg and 89 received AK112 20 mg/kg). As of Dec 31, 2021, in cohort-1, among 26 evaluable pts with squamous cell carcinoma, 20 partial response and 6 stable disease were observed for a 76.9% ORR and a 100.0% DCR, median DOR and median PFS was not reached while 6-month PFS rate was 86.2%. In Cohort-2, among 19 evaluable pts, 13 partial response and 5 stable disease were observed for a 68.4% ORR and a 94.7% DCR while median DOR was 5.5 months, and median PFS was 8.3 months. In Cohort-3, among 20 evaluable pts, 8 partial response and 8 stable disease were observed for a 40.0% ORR and a 80.0% DCR, median DOR and median PFS was not reached while 6-month PFS rate was 71.1%. Treatment emergent adverse events (TEAE) occurred in 86.5% (115/133) of the pts, and grade ≥3 AEs occurred in 28.6% (38/133) of pts including two deaths. Most common AE (incidence ≥ 5%) included alanine/aspartate aminotransferase increased, epistaxis, anemia, vomiting, nausea, rash, leukopenia, thrombocytopenia, and neutropenia. Treatment discontinuation due to AE occurred in 3.0% (4/133) of the pts.
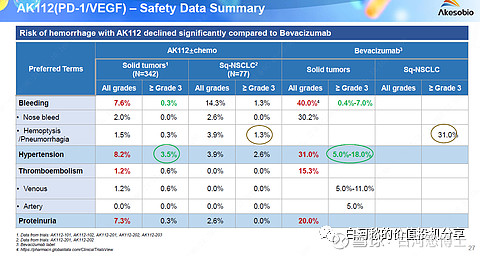
康方PD-1/VEGF双抗对一线PD-1联合化疗治疗进展20个人治疗的ORR为40%,且副作用比贝伐珠小.
信达:
IBI319
At cutoff date of February 10, 2022, 21 pts were enrolled (median age 55 years; 81.0% male; 19.1% ECOG PS 1; 52.4% advanced solid tumors). The following dose levels have been evaluated; 0.03 mg; 0.1 mg; 0.3 mg; 1 mg; 3 mg and 6 mg. Dose escalation is ongoing. The maximum tolerated dose has not been reached. Treatment-emergent adverse events (TEAEs) was reported in 15 out of 21 (71.4%) pts, among which 1 pts had back pain and nausea of grade 3. The most common TEAEs ( > 10%) were interleukin level increased (6 pts, 28.6%) and C-reactive protein increased (3 pts, 14.3%). Eleven pts (52.4%) experienced treatment-related AEs; none were grade 3 or higher. The most common TRAEs ( > 10%) were interleukin level increased (6 pts, 28.6%) and C-reactive protein increased (3 pts, 14.3%). Importantly, no drug-related elevations in transaminases (ALT, AST) or bilirubin have been seen. No DLT was observed. PK analysis of IBI319 demonstrated consistent exposure with linear PK. For 14 evaluable pts, 1 classical Hodgkin lymphoma patient achieved PR as best response.
IBI351
As of Feb 07 2022, 15 pts (13 men, 2 women; median age: 62 yrs, range: 48–74 yrs) were enrolled, among whom 12 had non-small cell lung cancer (NSCLC), and 3 had colorectal cancer (CRC). 4 pts had ≥3 prior lines of treatment (tx). Median tx duration was 66.5 ds (range: 21–98 ds). No dose-limiting toxicity (DLT) or any ≥grade 3 treatment-related adverse events were observed in any dose cohorts. A total of 12 patients (80.0%) had treatment-related adverse events (grade 1, n = 6; grade 2, n = 6). By investigator-assessment, tumor response was evaluated in 9 pts (4 with ≥2 assessments); 6 pts had not reached their first assessment. 2 pts had PR (1 NSCLC at wks 12, 450mg, tx ongoing; 1 CRC at wks 6, 700mg, tx ongoing), 4 pts (NSCLC) had SD, and 3 pts had PD (1 NSCLC at wks 12, 2 CRC at wks 6). As data cut-off date, 11 pts were continuing to receive IBI351 (GFH925).
IBI110
As data cutoff January 20th 2022, 18 patients were enrolled, 6 patients had locally advanced/recurrent tumors, and 12 had metastatic tumors. The median follow up time was 4 weeks (range, 0-20). The median exposure of combination therapy was 9.4 weeks (range, 3-24). Any grade TRAEs occurred in 16 pts (88.9%); most commonly white bloo4d cell count decreased (n = 6; 33.3%), neutrophil count decreased (n = 6; 33.3%), and aspartate aminotransferase increased (n = 6; 33.3%). Grade ≥ 3 TRAEs occurred in 5 pts (27.8%), including neutrophil count decreased (n = 2; 11.1%), platelet count decreased (n = 2; 11.1%) and hepatic function abnormal (n = 2; 11.1%), platelet count decreased (n = 2; 11.1%) and hepatic function abnormal (n = 2; 11.1%). Immune-mediated AEs occurred in 7 pts (38.9%); most commonly amylase increased (n = 2; 11.1%). One patient discontinued treatment because of the coronary artery disease. There were no treatment-related death. For 15 evaluable patients, the ORR and DCR were 60% (9 PR) and 100% (9 PR,6 SD), respectively. The median duration of response (DOR) and median progression free survival (PFS) were not reached. As data cutoff, 17 patients were still in treatment.
IBI110 (anti-LAG-3 mAb)
Totally, 20 patients were enrolled (median age: 63 [range: 52-74]; male: n = 19; ECOG 1: n = 16). As of data cutoff date, Jan 20, 2022, median follow up was 3.3 months (range: 2.6-7.0). The median exposure of combination therapy was 15.1 weeks (range: 6-52). The objective response rate was 80% (16/20, 9 patients with ≥2 efficacy assessments were confirmed PR and 7 needed further confirmation). The median progression-free survival and overall survival were not reached. The most common treatment-related adverse events (TRAEs) included white blood cell count decreased (50%), alopecia (50%), anaemia (45%), asthenia (40%), neutrophil count decreased (35%), rash (35%), and hyperglycaemia (30%); most common TRAEs ≥ grade 3 were neutrophil count decreased (30%), and white blood cell count decreased (20%). Immune-related AEs occurred in 11 patients (55%) and most were grade 1-2. The biomarker analysis including LAG-3 and PD-L1 expression in tumor specimen was ongoing.