有实力走出去的才是强者
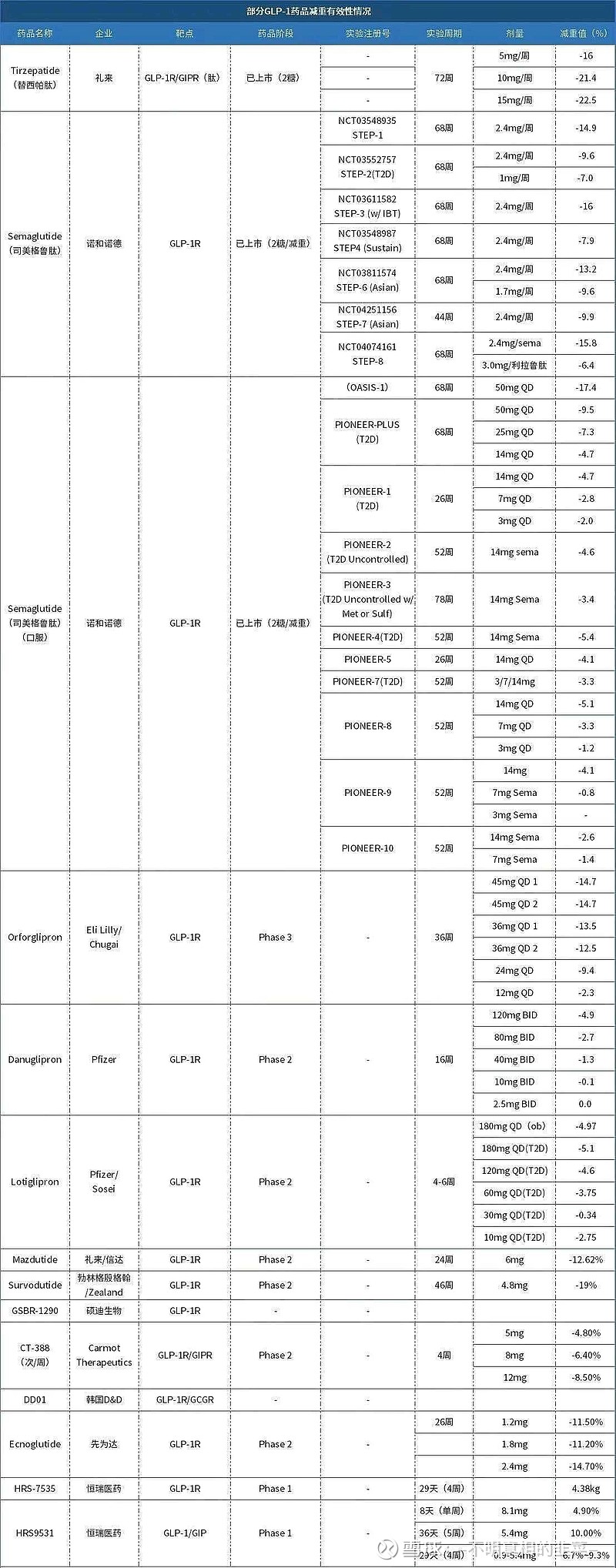
It remains unclear whether the superior efcacy of multi-target incretin analogs versus single-target incretins in obesity treatment. This randomized, double blind, placebo-controlled, dose-escalation phase Ib/la study aimed to assess the efficacy and safety of a GLP-1 analog, GZR18, in Chinese adults with obesity. The study investigated the weight loss potential of GZR18 and evaluated the feasibility of administrating GZR18 at different frequencies. Thirty-six participants with obesity were randomized 3:1 to recelive 30 mg of GZR18 or a placebo for 35 weeks, including a 31-week dose-escalation period. Upon dose escalation to 9 mg/week, subjects were divided into dosing sub-cohorts of QW or Q2W. Endpoints were body weight change and AEs incidence. The average weight loss of GZR18 adjusted by placebo was 18.6% in QW group and 13.5% in Q2W group, with no IP-related serious AEs. Gastrointestinal AEs were reported most frequently, mainly in early dose-escalation period. GZR18 reduced body weight robustly and improved metabolic profiles in study participants. Its weight-loss effects surpassed those of Semaglutide (2.4 mg) and Tirzepatide (15 mg) In recent phase 3 trials involving similar Chinese populations (-9.8% and -17.5%。respectively). These findings warrant further investigation into GZR18's potential to offer superior weight management eficacy over multi-target incretin analogs.目前尚不清楚多靶点肠促胰岛素类似物在肥胖治疗中是否优于单靶点肠增胰岛素。这项随机、双盲、安慰剂对照、剂量递增期Ib/la研究旨在评估GLP-1类似物GZR18在中国肥胖成年人中的疗效和安全性。本研究调查了GZR18的减肥潜力,并评估了在不同频率下给予GZR18治疗的可行性。36名肥胖参与者以3:1的比例随机接受30 mg GZR18或安慰剂治疗35周,包括31周的剂量递增期。当剂量增加到9 mg/周时,受试者被分为QW或Q2W的给药亚组。终点是体重变化和不良事件发生率。经安慰剂调整的GZR18的平均体重减轻在QW组为18.6%,在Q2W组为13.5%,没有IP相关的严重不良事件。胃肠道不良事件报告最频繁,主要发生在早期剂量增加期。GZR18稳定地减轻了研究参与者的体重,改善了他们的代谢状况。在最近涉及类似中国人群的3期试验中,其减肥效果超过了诺和诺德的司美格鲁肽(2.4 mg)和礼来制药的替尔帕肽(15 mg)(分别为-9.8%和-17.5%)。这些发现值得进一步研究GZR18在提供优于多靶点肠促生长素类似物的体重管理效率方面的潜力。