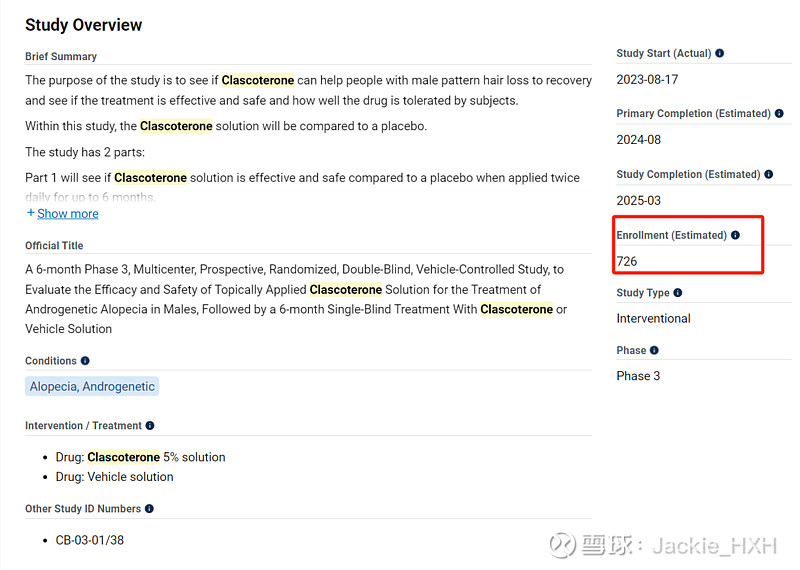
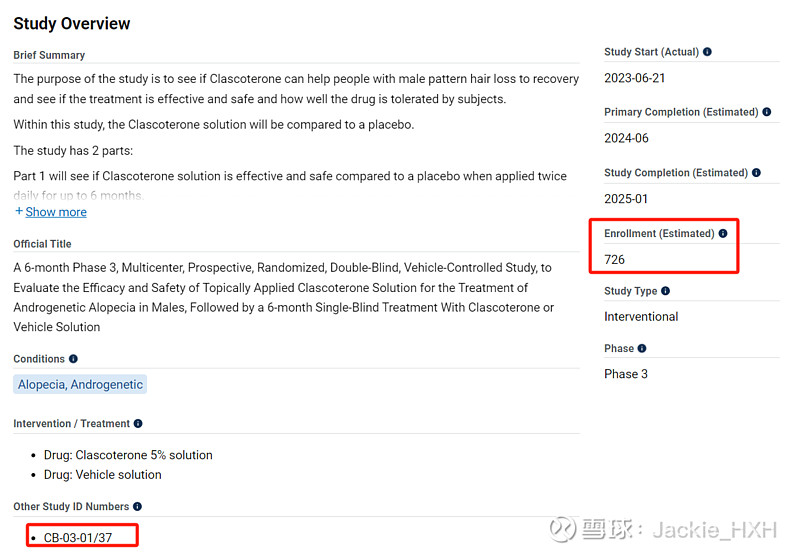
$三生制药(01530)$ 痤疮药(乳膏剂)的合作伙伴开发的同成分不同剂型(溶液剂)雄秃药物两个三期临床进行中,且都已入组大半。
6个月的临床数据预计今年6月份或者8月份拿到。如果积极就只需要对有反应的患者再等6个月的追加临床时间以便获得治疗维持效果。
从国外自免类2个3期临床的标准打法,感觉能成的概率蛮高的。
三生对这款雄秃药目前拥有优先购买权。
Breezula® (our drug for the treatment of androgenetic alopecia) phase III studies in males commenced in June 2023 and is currently ongoing in the U.S. In the EU, study authorisation was received in November and recruitment has commenced as well. To date, 855 patients in total (348 in study CB-03-01/37 and 507 in study CB-03-01/38) have been randomized and started the treatment. An estimated 2 billion males worldwide are affected by androgenetic alopecia including 80-90 million males in the U.S. only, of which only a negligible portion are seeking treatment.