IHS Markit COVID-19 Research and Development Update
Gustav Ando, head of life sciences and industry services, IHS Markit
Janet Beal, senior life sciences analyst, IHS Markit
IHS Markit forecasts that a fully approved, effective vaccine may not be available until the summer of 2021
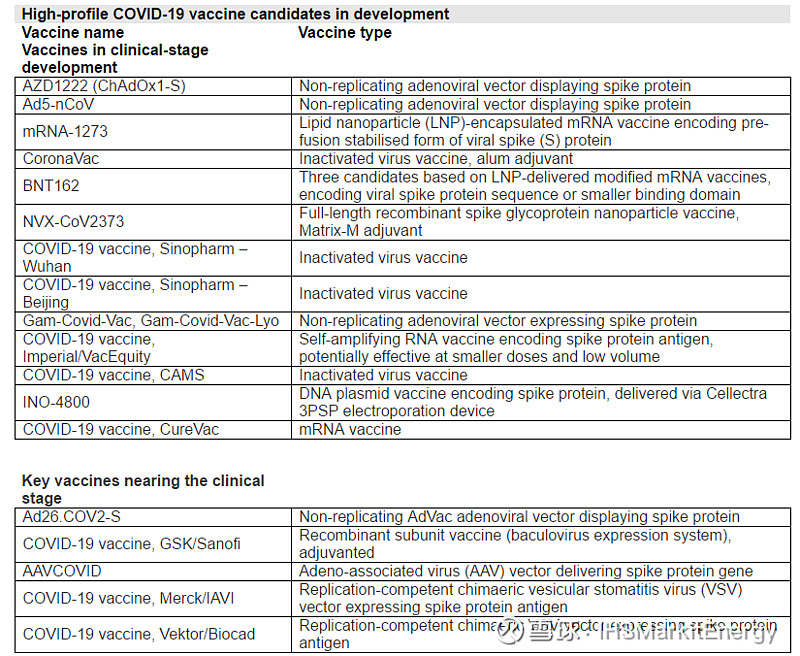
Thirteen COVID-19 vaccine candidates are currently in clinical trials (as of 18 June). These are listed in the table below, in approximate descending order of advancement, taking into account scale of development and expected reporting dates. In addition, selected promising preclinical-stage projects (some very close to initiating clinical development) have also been listed.
Latest developments for vaccines in the week ending 18 June:
Chinese firm Sinovac has announced a major collaboration with Instituto Butantan (Brazil) for advancement of its vaccine candidate CoronaVac into Phase III testing in Brazil and China. In addition, Sinovac has reported preliminary Phase I/II results for CoronaVac from 743 patients, showing initial signs of immunogenicity and neutralising antibodies, with no safety concerns.
A Phase I trial has started for German firm CureVac’s mRNA vaccine candidate in Belgium and Germany. Just two days before the start of this trial, the German government acquired a 23% stake in CureVac.
The first Russian COVID-19 vaccine candidate has formally entered Phase I/II trials. An adenovirus-based product in two forms, Gam-Covid-Vac (solution for injection) and Gam-Covid-Vac-Lyo (lyophilised powder for solution preparation), has been developed by the state Gamalei Institute, in partnership with Russian manufacturer R-Pharm.
Imperial College London, in partnership with VacEquity Global Health (VGH), has initiated a Phase I/II trial of a self-amplifying RNA-based UK candidate vaccine, which uses an mRNA-based rationale, but may be effective at extremely small doses, and would have considerably reduced manufacturing volume requirements, if successful.
“
“Unusually, several companies developing promising vaccine candidates have embarked on plans for at-risk high-level manufacture. It has been generally recognised that, if and when a suitably effective and safe vaccine is approved, stocks will need to be made available as rapidly as possible to immunise the population. Given the urgency of the situation, emergency authorisations may become feasible in certain territories before formal approval takes place. In view of this necessity for scale-up, a growing number of smaller biotechs and academic groups developing the more innovative candidates have secured major partnerships with experienced players in this territory."
– Gustav Ando, head of life sciences and industry services, IHS Markit
”
A fully approved, effective treatment against COVID-19 will likely not be available until the first half of 2021
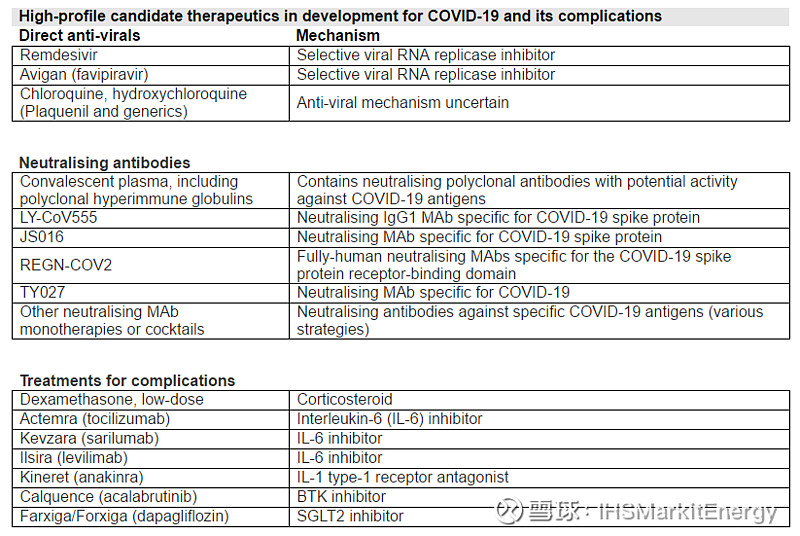
In the absence of a vaccine, effective therapeutics for COVID-19 and its complications are urgently being sought to help mitigate the most serious consequences of infection and to reduce the duration of virus shedding and infectivity. This is especially important since the timeline for approval of a repurposed known medicine would be much shorter than that for a vaccine, since basic safety should already be established. IHS Markit has evaluated current evidence and status for a selection of promising medicines, together with current high-profile anti-virals, that are under evaluation for COVID-19 infection and its complications (see table below).
Latest developments for COVID-19 therapeutics in the week ending 18 June:
A low-cost and readily available medicine, low-dose dexamethasone, has shown preliminary evidence of a major effect in reducing mortality risk in critically ill COVID-19 patients requiring oxygen. Results from the large UK RECOVERY adaptive trial have indicated a reduction in mortality risk versus standard care of up to 35% in patients requiring ventilation and up to 20% in those requiring oxygen only, although no benefit was indicated in less serious patients. As a result of these preliminary data, the UK government has already adopted this treatment as part of NHS standard care for serious COVID-19 with immediate effect and has placed a parallel export ban on dexamethasone. These preliminary findings have also been submitted to the WHO for evaluation.
The WHO has halted the hydroxychloroquine arm of the SOLIDARITY trial for a second time, following analysis of data submitted from the major UK RECOVERY trial indicating little benefit or reduction in mortality in COVID-19. Meanwhile, in the UK, the Medicines and Healthcare Products Regulatory Agency (MHRA) has ordered that no new participants should be recruited into any major ongoing UK clinical trials evaluating hydroxychloroquine.
The US FDA has revoked its EUA for chloroquine and hydroxychloroquine, which had facilitated the donation of the treatments from the Strategic National Stockpile to help treat hospitalised COVID-19 patients when clinical trial enrolment was not available or feasible. The decision was made because the “legal criteria for issuing an EUA are no longer met”, and the FDA considered these drugs were “unlikely to be effective in treating COVID-19” in hospitalised patients. The agency also noted that the potential benefits of the treatment no longer outweigh the potential risks, including cardiotoxicity and other side effects of the treatment.
The FDA has also warned healthcare providers about potential drug interactions between hydroxychloroquine/chloroquine and remdesivir in hospitalised patients with severe COVID-19.
Regeneron has initiated the first clinical trial for a neutralising antibody cocktail. REGN-COV2 (REGN10933 + REGN10987) will be tested in adaptive Phase I/II/III trials in four study populations, including hospitalised and non-hospitalised symptomatic patients, uninfected high-risk groups, and uninfected people post-exposure, for prevention and treatment of COVID-19. The cocktail contains two non-competing neutralising antibodies binding to the receptor binding domain (RBD) of the spike protein, designed to avoid viral escape. Regeneron used a similar strategy to develop a triple antibody candidate for Ebola.
Gilead Sciences has announced plans to conduct a Phase II/III trial of remdesivir in paediatric moderate-to-severe COVID-19 patients, including newborns, in Europe and the US.
“
“Development of neutralising antibodies is in some ways more promising, but human efficacy data will be crucial. The most advanced explorations of this type of treatment have involved the use of convalescent plasma from recovered COVID-19 donor patients – an intervention that is in a large number of trials around the world currently, mostly government- or hospital-sponsored. Positive results from this type of intervention should pave the way for more targeted versions of the same strategy – the use of cocktails of selected neutralising antibodies to reduce the viral load in patients with severe disease, or even a single agent.”
– Gustav Ando, head of life sciences and industry services, IHS Markit
”
Excerpts from the IHS Markit COVID-19 Vaccine and Treatment Update follow. Please feel free to quote from above with attribution.
This tracker is designed to highlight the candidates for treatments and vaccines against coronavirus disease 2019 (COVID19) that IHS Markit considers the most important for the market to watch.
For additional analysis of phases of vaccines and treatments or other media inquiries contact: press@ihsmarkit.com
Free access: Industry-leading healthcare industry intelligence and up-to-date COVID-19 insights
Register today to receive access to the most intelligent and timely data and expert coverage of the healthcare aspects of COVID-19, including the risks of second and third waves, development of vaccines/treatments, and government/policy response.
We are currently offering free access to our analysis with no obligation to subscribe.
To access the World Markets Healthcare service's trial, please provide your details, and our team will contact you shortly upon submission.
点击“此处”,填表申请免费使用。
埃信华迈(IHS Markit,纽约证交所股票代码:INFO)是信息处理、研究咨询领域的全球先进企业。为能源及自然资源产业链(包括上游、石油产业链以及化工、天然气、电力以及新能源等),海陆空交通,科技及金融等主要产业和市场提供专业数据,软件以及咨询和研究分析服务。为客户做出更明智和自信的决策提供依据,帮助客户实现业务增长和效率提升。埃信华迈拥有50,000多家政府及企业客户,包括全球财富500强中80%的企业,以及众多世界先进的金融机构。埃信华迈公司注册于英属百慕大,总部设在伦敦,并致力于可持续的盈利性增长。
如您想获得更有关于其他能源的最新信息,请通过电子邮件,把您的要求发送给我们。
了解最新的能源洞察
网页链接
联系我们
ENRChina@ihsmarkit.com
点击“此处”,填表申请免费使用。