$百济神州-U(SH688235)$ $百济神州(06160)$
百济在EHA公布泽布替尼联合BGB-11417在RR-CLL疗效数据
泽布替尼联合BGB-11417在RR-CLL上展现深度持续性疗效反应并且安全性目前看起来还不错
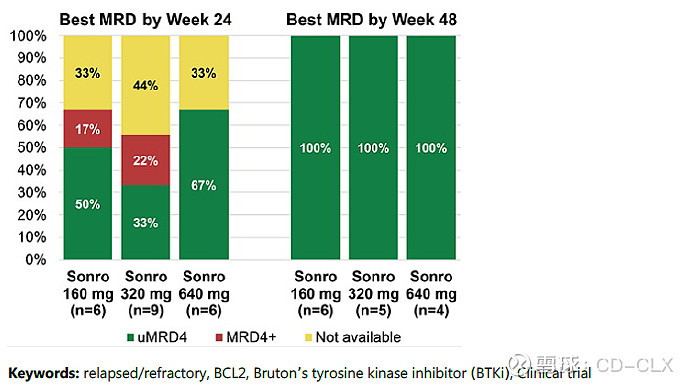
ORR=97%; CR%=50%;治疗达到48的受试者都达到了uMRD; RP2D 320 mg;
Title: RESULTS FROM THE PHASE 1 STUDY OF THE NOVEL BCL2 INHIBITOR SONROTOCLAX (SONRO) IN COMBINATION WITH ZANUBRUTINIB (ZANU) FOR RELAPSED/REFRACTORY (R/R) CLL/SLL SHOW DEEP AND DURABLE RESPONSES
Abstract Type: Oral Presentation
Session Title: Novel therapies in relapsed and refractory CLL and hairy cell leukemia
Background:
Despite recent therapeutic advances, CLL/SLL remains incurable. The majority of treated patients relapse, necessitating further treatment with novel agents. BCL2 inhibition is an established therapeutic strategy in CLL/SLL, and the addition of BTK inhibition is synergistic. Sonro (BGB-11417), a next-generation BCL2 inhibitor, is a more selective and potent inhibitor of BCL2 than venetoclax in biochemical assays. Zanu, a next-generation BTK inhibitor (BTKi), has shown improved PFS and tolerability, including fewer cardiac AEs than ibrutinib in a randomized study of patients with R/R CLL/SLL and is approved for the treatment of CLL.
Aims:
To report updated safety and efficacy data for patients with R/R CLL/SLL treated with sonro + zanu in the ongoing BGB-11417-101 (NCT04277637) study.
Methods:
Patients with R/R CLL/SLL received zanu (320 mg QD or 160 mg BID) 8-12 wk before starting sonro (40, 80, 160, 320, or 640 mg QD) with ramp-up to the target dose to mitigate potential risk of tumor lysis syndrome (TLS). Prior BTKi was allowed if disease progression occurred on treatment. Patients were treated until disease progression or unacceptable toxicity. The primary endpoint was safety (per CTCAE v5.0); ORR (per iwCLL 2008 criteria) and minimal residual disease assessed in blood by ERIC flow every 24 weeks (uMRD4) were secondary and exploratory endpoints, respectively.
Results:
As of Oct 31, 2023, 45 patients with R/R CLL/SLL were enrolled across different cohorts (40 mg, n=4; 80 mg, n=9; 160 mg, n=6; 320 mg, n=20; 640 mg, n=6). Four patients were still in the zanu lead-in phase and 41 had started sonro. The median age was 65 yrs (range, 36-76 yrs); 28% of tested patients (11/40) had del(17p), and 72% (13/18) had unmutated IGHV. The median number of prior treatments was 1 (range, 1-3); 7 patients had prior BTKi as their last therapy. The median follow-up was 17 months (range, 0.5-32.6 months). There were no DLTs; up to 640 mg, the MTD had not been reached. Dose expansion was completed with a recommended phase 2 dose of 320 mg. Any-grade treatment-emergent AEs (TEAEs) that occurred in ≥20% of patients were COVID-19 (n=12; 27%), contusion (n=12 [27%]), neutropenia (n=12 [27%]), diarrhea (n=11 [24%]), nausea (n=11 [24%]) and fatigue (n=11 [24%]). Neutropenia was the most common grade ≥3 TEAE (n=9 [20%]). No cases of TLS or atrial fibrillation occurred; no TEAEs led to death, discontinuation, or dose reduction. Sonro dose holds occurred in 14 patients for a median duration of 7 days, most commonly due to COVID-19 (n=7). For 32 response-evaluable patients across all dose levels, the ORR was 97% (31/32; 1 patient [40 mg] had SD), and the complete response (CR) rate was 50% (40 mg, n=1 [25%]; 80 mg, n=4 [50%]; 160 mg, n=4 [67%]; 320 mg, n=5 [56%]; 640 mg, n=2 [40%]). The median time to CR was 9.8 months (range, 5.5-18.2 months). Of 4 response evaluable patients with prior BTKi treatment, 3 achieved PR (n=2) or CR (n=1). All patients treated with sonro +zanu (160 mg, 320 mg, or 640 mg) who reached week 48 achieved uMRD4 (Figure). Treatment is ongoing for all but 1 patient in the 40 mg cohort who discontinued due to disease progression.
Summary/Conclusion:
Efficacy of sonro + zanu combination treatment is encouraging, with a 97% ORR and deep responses, including uMRD, in patients with R/R CLL/SLL. This combination has demonstrated a tolerable safety profile across all dose levels tested.