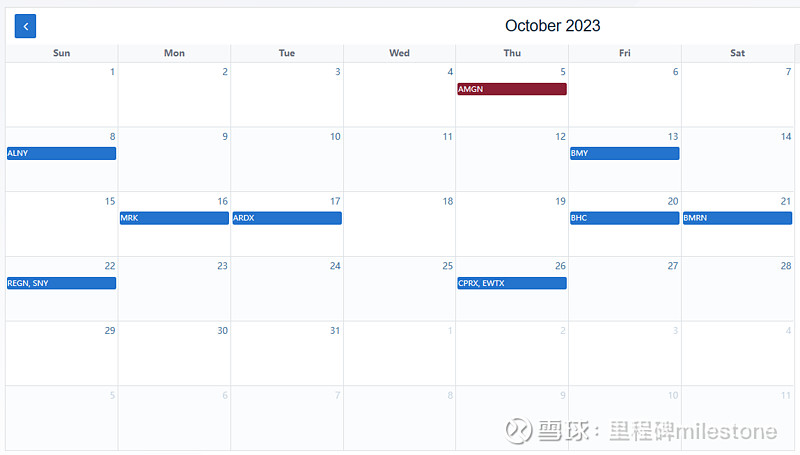
17/10/2023 ET XPHOZAH (tenapanor) $Ardelyx(ARDX)$ $4.82 NDA resubmitted April 18, 2023. FDA issued CRL on July 29 2021. Advisory committee recommended approval of tenapanor, noted November 16, 2022. Appeal for CRL granted December 29, 2022. NDA resubmission accepted by FDA, noted May 17, 2023. PDUFA date assigned within 6 months since resubmission to October 17, 2023. 网页链接
20/10/2023 ET IDP-126 $Bausch Health(BHC)$ $8.46 PDUFA date on October 20, 2023. 网页链接
21/10/2023 ET VOXZOGO (Vosoritide) $拜玛林制药(BMRN)$ $93.03 PDUFA data of October 21, 2023. 网页链接