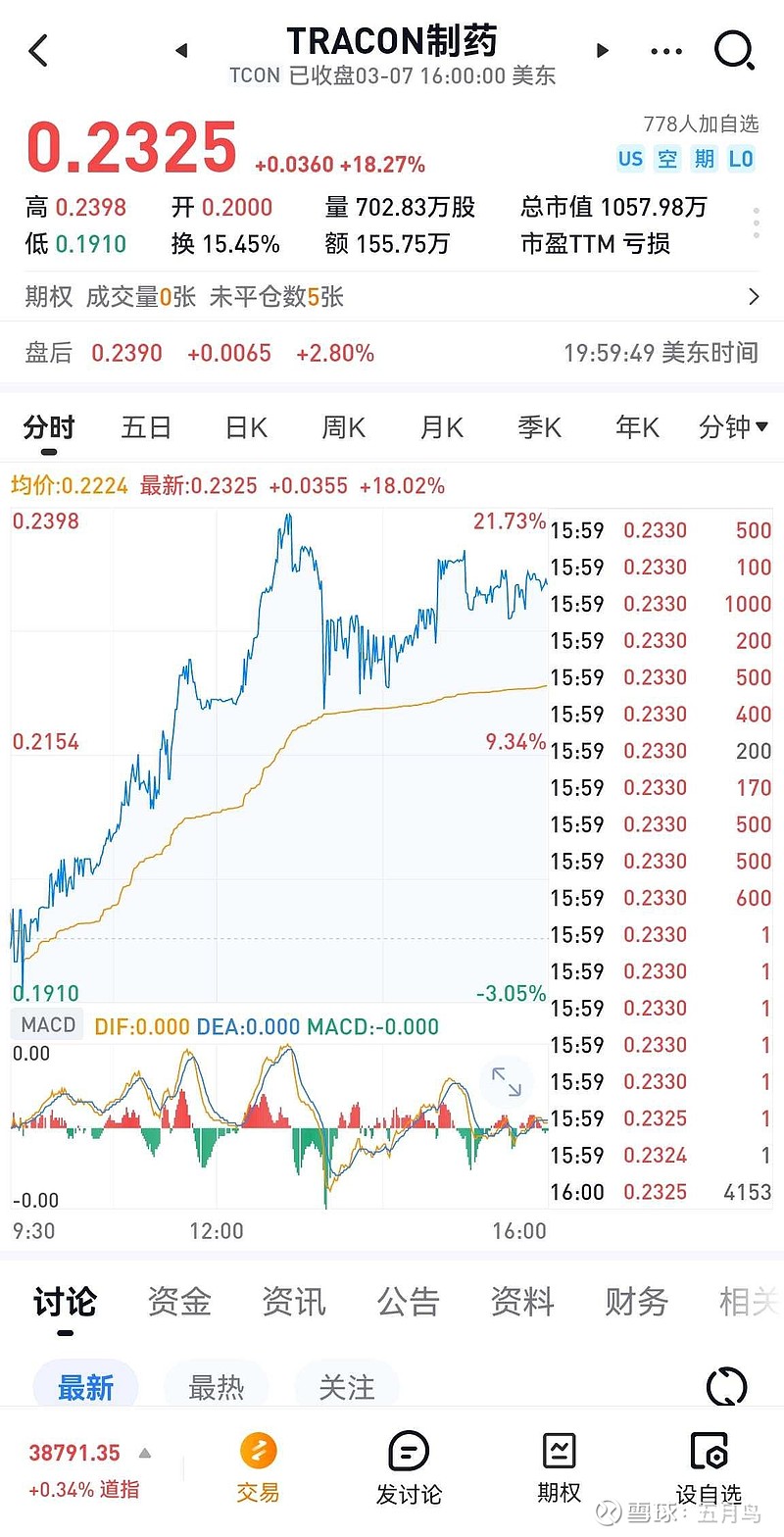

| 发布于: | Android | 转发:0 | 回复:8 | 喜欢:0 |

现金流枯竭,035美国临床做出低效,ORR远低于国内数据,公司基本退市倒闭成定局。
12月,我们宣布了ENVASRC 2期关键试验的最新中期数据
最初的46名患者接受了单剂envafolimab治疗。客观反应率
(ORR)在研究者评审中为15%,在盲法独立中心评审中为8.7%
(BICR),所有这些都是已确认的应答。Envafolimab单药治疗总体良好
BICR的耐受性和中位反应持续时间大于6个月。初级
该研究的终点是80名患者中有9名(11.25%)通过BICR实现ORR
接受envafolimab治疗,中位反应持续时间超过6个月是关键
次要终点。
Additional safety and efficacy data were reviewed for 46 patients enrolled into cohort C who were the subject of the September independent data monitoring committee (IDMC) review. At that time, patients had completed a minimum of 12 weeks of efficacy evaluations and the objective response rate (ORR) was 13% by investigator review and 8.7% by blinded independent central review (BICR). Since then, an additional patient has achieved an objective response by investigator review, which increased the ORR by investigator review to 15%. The most recent objective response has not yet been confirmed by BICR and the patient remains on treatment. Median duration of response by BICR remains greater than six months. In addition, envafolimab remains well tolerated and grade > 3 related toxicity has not been reported to date.
总市值七千多万人民币
有确切消息吗?
035在国内也算卖的好的PD-(L)1了