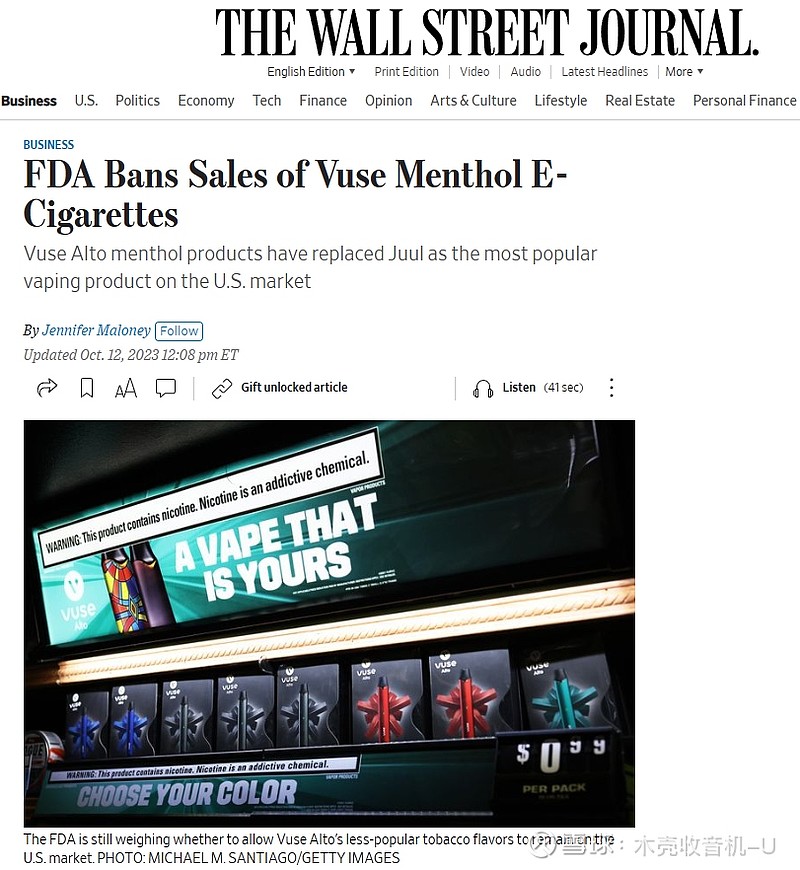
The Food and Drug Administration ordered Reynolds American to stop selling menthol-flavored versions of its Vuse Alto e-cigarette—the top-selling vaping brand in the U.S. and one that has become popular among children and teenagers.
The agency on Thursday said that Reynolds failed to demonstrate that the products presented enough of a public-health benefit to adult cigarette smokers to outweigh the risk to youth. The federal regulator is still weighing whether to allow Vuse Alto’s less-popular tobacco flavors to remain on the U.S. market.
Vuse Alto products represented about 40% of U.S. e-cigarette sales in U.S. retail stores in the 52 weeks ended Sept. 23, according to an analysis of Nielsen data by Goldman Sachs analyst Bonnie Herzog. Menthol is by far the brand’s most popular flavor, representing $1.6 billion, or about 29%, of e-cigarette sales in U.S. stores in the past year, Herzog said. The data don’t include vape shops or online sales.
Juul, once the market leader, is now the No. 2 U.S. vaping brand. Its tobacco and menthol flavors combined represented about 25% of U.S. e-cigarette sales in stores tracked by Nielsen.
Vuse Alto’s tobacco flavors represented 9% of the U.S. e-cigarette market, Herzog said. The FDA rejected Reynolds’s application to bring Alto’s mixed-berry flavor back onto the market. In an effort to curb underage vaping, the U.S. in 2020 halted sales of all sweet and fruity e-cigarette refill cartridges such as those made by Vuse.
Reynolds, a subsidiary of British American Tobacco, also makes Camel and Newport cigarettes. Like other tobacco giants, BAT has been trying to pivot to e-cigarettes and other tobacco alternatives as cigarette smoking rates decline. The company is also bracing for a planned U.S. ban on menthol cigarettes.
All U.S. e-cigarette manufacturers in 2020 were required to submit their vaping products for FDA authorization. Some of those applications are still under review. The agency has been weighing, among other things, the potential public-health benefit of adult smokers switching to a safer option against the potential harm of young people getting hooked on nicotine.
The FDA last year briefly ordered Juul Labs to halt sales of its vaping products, citing unresolved technical questions in the company’s application. The agency then put the ban on hold while Juul appealed. That appeal is pending.
Federal survey data released last year showed that Vuse’s popularity among young people was climbing: Some 12.5% of middle- and high-school students who had vaped in the past 30 days said Vuse was their usual brand, making it the second-most popular brand among children and teens behind Puff Bar, according to the survey.
Write to Jennifer Maloney at Jennifer.Maloney@wsj.com